Width: 0px, Height: 0px
Width: 1197px, Height: 997px
Width: 1158px, Height: 965px
Width: 1123px, Height: 936px
Width: 1093px, Height: 911px
Width: 1065px, Height: 888px
Width: 1043px, Height: 869px
Width: 1033px, Height: 861px
Width: 1026px, Height: 855px
Width: 1018px, Height: 848px
Width: 1015px, Height: 846px
Width: 1003px, Height: 836px
Width: 996px, Height: 830px
Width: 992px, Height: 827px
Width: 987px, Height: 822px
Width: 986px, Height: 822px
Width: 985px, Height: 821px
Width: 983px, Height: 819px
Width: 982px, Height: 818px
Width: 981px, Height: 817px
Width: 980px, Height: 817px
Width: 978px, Height: 815px
Width: 972px, Height: 810px
Width: 966px, Height: 805px
Width: 953px, Height: 794px
Width: 946px, Height: 788px
Width: 940px, Height: 783px
Width: 928px, Height: 773px
Width: 916px, Height: 763px
Width: 895px, Height: 746px
Width: 881px, Height: 734px
Width: 872px, Height: 727px
Width: 862px, Height: 718px
Width: 852px, Height: 710px
Width: 842px, Height: 702px
Width: 830px, Height: 692px
Width: 820px, Height: 683px
Width: 808px, Height: 673px
Width: 805px, Height: 671px
Width: 787px, Height: 656px
Width: 778px, Height: 648px
Width: 773px, Height: 644px
Width: 767px, Height: 639px
Width: 762px, Height: 635px
Width: 761px, Height: 634px
Width: 758px, Height: 632px
Width: 756px, Height: 630px
Width: 749px, Height: 624px
Width: 746px, Height: 622px
Width: 742px, Height: 619px
Width: 739px, Height: 616px
Width: 738px, Height: 615px
Width: 736px, Height: 614px
Width: 734px, Height: 612px
Width: 732px, Height: 610px
Width: 731px, Height: 609px
Width: 731px, Height: 609px
Width: 731px, Height: 609px
Width: 729px, Height: 608px
Width: 726px, Height: 605px
Width: 724px, Height: 604px
Width: 722px, Height: 602px
Width: 719px, Height: 599px
Width: 718px, Height: 599px
Width: 718px, Height: 599px
Width: 717px, Height: 598px
Width: 714px, Height: 595px
Width: 714px, Height: 595px
Width: 711px, Height: 593px
Width: 711px, Height: 593px
Width: 709px, Height: 591px
Width: 708px, Height: 590px
Width: 707px, Height: 589px
Width: 704px, Height: 587px
Width: 701px, Height: 584px
Width: 699px, Height: 583px
Width: 698px, Height: 582px
Width: 692px, Height: 577px
Width: 691px, Height: 576px
Width: 689px, Height: 574px
Width: 688px, Height: 574px
Width: 688px, Height: 574px
Width: 688px, Height: 574px
Width: 687px, Height: 573px
Width: 686px, Height: 572px
Width: 684px, Height: 570px
Width: 684px, Height: 570px
Width: 683px, Height: 569px
Width: 683px, Height: 569px
Width: 683px, Height: 569px
Width: 683px, Height: 569px
Width: 682px, Height: 569px
Width: 681px, Height: 568px
Width: 677px, Height: 564px
Width: 673px, Height: 561px
Width: 672px, Height: 560px
Width: 668px, Height: 557px
Width: 667px, Height: 556px
Width: 667px, Height: 556px
Width: 666px, Height: 555px
Width: 664px, Height: 554px
Width: 663px, Height: 553px
Width: 661px, Height: 551px
Width: 659px, Height: 549px
Width: 658px, Height: 549px
Width: 658px, Height: 549px
Width: 658px, Height: 549px
Width: 657px, Height: 548px
Width: 656px, Height: 547px
Width: 654px, Height: 545px
Width: 653px, Height: 544px
Width: 651px, Height: 543px
Width: 649px, Height: 541px
Width: 648px, Height: 540px
Width: 647px, Height: 539px
Width: 646px, Height: 539px
Width: 646px, Height: 539px
Width: 644px, Height: 537px
Width: 643px, Height: 536px
Width: 642px, Height: 535px
Width: 641px, Height: 534px
Width: 641px, Height: 534px
Width: 641px, Height: 534px
Width: 639px, Height: 533px
Width: 639px, Height: 533px
Width: 638px, Height: 532px
Width: 637px, Height: 531px
Width: 636px, Height: 530px
Width: 634px, Height: 529px
Width: 632px, Height: 527px
Width: 631px, Height: 526px
Width: 629px, Height: 524px
Width: 628px, Height: 524px
Width: 626px, Height: 522px
Width: 626px, Height: 522px
Width: 626px, Height: 522px
Width: 626px, Height: 522px
Width: 626px, Height: 522px
Width: 624px, Height: 520px
Width: 623px, Height: 519px
Width: 622px, Height: 519px
Width: 621px, Height: 518px
Width: 619px, Height: 516px
Width: 618px, Height: 515px
Width: 618px, Height: 515px
Width: 617px, Height: 514px
Width: 614px, Height: 512px
Width: 614px, Height: 512px
Width: 614px, Height: 512px
Width: 611px, Height: 509px
Width: 609px, Height: 508px
Width: 609px, Height: 508px
Width: 609px, Height: 508px
Width: 609px, Height: 508px
Width: 607px, Height: 506px
Width: 607px, Height: 506px
Width: 607px, Height: 506px
Width: 606px, Height: 505px
Width: 606px, Height: 505px
Width: 603px, Height: 503px
Width: 603px, Height: 503px
Width: 603px, Height: 503px
Width: 603px, Height: 503px
Width: 603px, Height: 503px
Width: 603px, Height: 503px
Width: 602px, Height: 502px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 601px, Height: 501px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 599px, Height: 499px
Width: 601px, Height: 501px
Width: 602px, Height: 502px
Width: 603px, Height: 503px
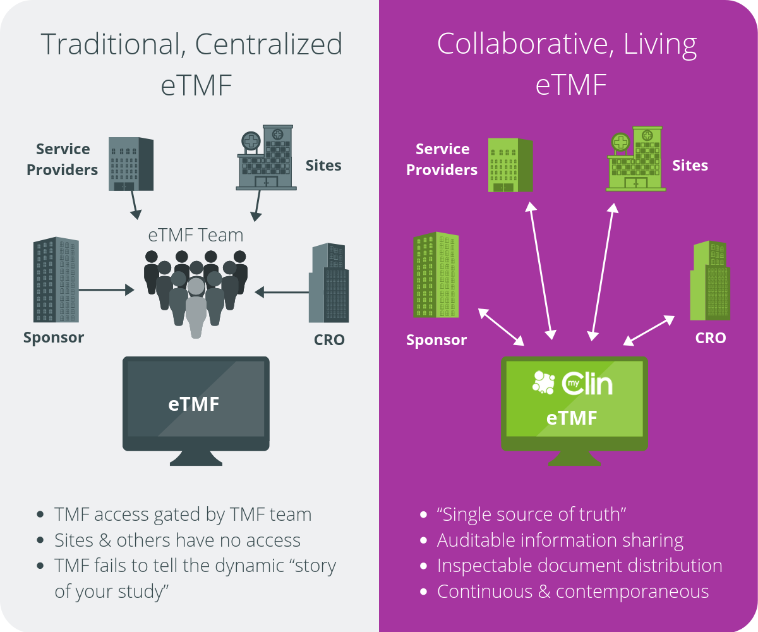
These trials generate a lot of paperwork—approvals, reports, agreements, and more. The eTMF keeps all of this in one secure, organized place, but in digital format.